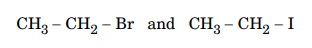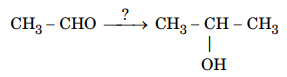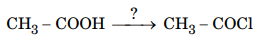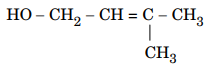1. The molecule, AB of a compound forms cubic lattice where ‘A’ atoms occupy the corners and B atoms occupy the alternate faces of the unit cell. The formula of the compound is :
(a) A2B
(b) AB2
(c) AB
(d) A2B2
2. For the given reaction 2A + B -> C + D, the rate constant is 1.5 × 10–4 s–1. What is the order of the reaction?
(a) one
(b) two
(c) zero
(d) three
3.Which of the following is isostructural with XeF4?
(a) ICl42–
(b) ICl4–
(c) SO42–
(d) SF4
4.What is the coordination number of Co in [Co(NO2)2 (en)2]+
(a) 4
(b) 5
(c) 6
(d) 3
5.C6H5NH2 and C6H5CH2NH2 can be distinguished by
(a) Fehling’s test
(b) Isocyanide test
(c) Adding NaNO2 + HCl
(d) Iodoform test
6. While antacids and antiallergic drugs interfere with the function of histamines, why do these not interfere with the function of each other?
................................ Advertisement ................................
7. What are antidepressants? Give an example.
8.A person suffers from RBC deficiency in haemoglobin. Which vitamin deficiency does the person has? Name two food items which should be included in the diet to restore the health back to normal?
9.Why two strands of DNA are not identical but are complimentary to each other?
10.Name the products of hydrolysis of Protein.
11.Name the products of hydrolysis of Nucleotide.
12.Name the products of hydrolysis of Lactose.
13. Gabriel phthalimide synthesis is preferred for preparation of primary amines. Show equation for the reactions involved.
14.Explain why pKb of methanamine is 3.38 while that of aniline is 9.38.
15.Transition metals have high enthalpy of atomization.Give reason.
16.Name a compound where the transition metal is in the +7 oxidation state.
................................ Advertisement ................................
17.Name a transition metal which does not exhibit variation in oxidation state in its compounds.
18.Mn2O7 is acidic whereas MnO is basic. Give reason.
19.Which would undergo SN2 reaction faster in the following pair and why ?
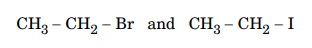
20. Mn2+ compounds are more stable towards oxidation than Fe2+ compounds.Give reason.
21. Cu2+ ion is coloured while Zn2+ ion is not.Give reason.
22.Why are lyophilic colloidal sols more stable than lyophobic colloidal sols?
23.What form Freundlich adsorption equation will take at high pressure?
24.Name the Ore used in the preparation of Potassium dichromate.
................................ Advertisement ................................
25.Name a member of the lanthanoid series which is well known to exhibit +4 oxidation state.
26.How will you prepare HBr from NaBr ?
27.How will you prepare SO2 from H2SO4?
28.How will you prepare XeO3 from XeF6?
29.Though copper has completely filled d-orbital (d10) yet it is considered as a transition metal. Give reason.
30.Actinoids show wide range of oxidation states. Give reason.
................................ Advertisement ................................
31.Write the equations involved in the preparation of Potassium dichromate from Sodium chromate (Na2CrO4).
32.What happens when phenol reacts with Bromine water ?
33.What happens when ethanol reacts with CH3COCl/pyridine ?
34.What happens when anisole reacts with HI ?
35.HClO4 is a stronger acid than HClO.Why?
36.Give IUPAC name of the ionisation isomer of [Cr(NH3)5Br]SO4.
37.Draw the structures of optical isomers of [CoBr2(Ox)2]3–.
38.Identify A and B in the foll reaction:
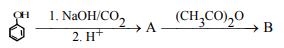
39.Limestone is used in the manufacture of pig iron from haematite.Give reason.
40.The reduction of a metal oxide is easier if the metal formed is in the liquid state at the temperature of reduction.Give reason.
................................ Advertisement ................................
41.What happens when a freshly precipitated Fe (OH)3 is shaken with a little amount of dilute solution of FeCl3?
42.Identify A and B in the foll reaction:
43.Dinitrogen is chemically inert.Give reason.
44.Why is common salt used to clear the snow off the road?
45.Give an example of a substance that can act as a disinfectant as well as antiseptic depending upon its concentration. (Specify concentration)
46.Name any two macromolecules chosen as drug targets.
47.Pick out the odd one from among the following on the basis of their medicinal properties mentioning the reason: Luminal, Seconal, Phenacetin, Equanil.
48.Explain why the dipole moment of chlorobenzene is lower than that of cyclohexyl chloride.
................................ Advertisement ................................
49.Pine oil is used in the froth floatation process used to concentrate sulphide ores.Give reason.
50.One gram of pulverised wood burns faster than one gram piece of wood.Give reason.
51.PCl5 acts as an oxidising agent.Give reason.
52. An increase of 10K in temperature rarely doubles the kinetic energy of particles but this much increase in temperature may be enough to double the rate of reaction.Explain.
53.Propanone is less reactive than ethanol toward addition of HCN.Give reason.
54.How can you convert Toluene to Benzaldehyde?
55.How can you convert Ethanoic acid to 2-chloroethanoic acid ?
56.How can you convert Acetone to Propane ?
................................ Advertisement ................................
57.Define the term Sorption.
58.Define the term Zeta potential.
59.Define the term Kraft temperature.
60.Name the non stoichiometric point defect responsible for colour in alkali metal halides.
61.Hydrogen fluoride is a weaker acid than hydrogen chloride in aqueous solution.Give reason.
62.PCl5 is ionic in nature in the solid state.
63.SF6 is inert towards hydrolysis.
65.Out of noble gases only Xenon is known to form established chemical compounds.Give reason.
66.Draw the structure of the following derivative: The 2,4-Dinitrophenylhydrazone of benzaldehyde.
67.Draw the structure of the following derivative: Acetaldehydedimethyl acetal.
68.Draw the structure of the following derivative:Cyclopropanone oxime.
69.What is shape selective catalysis?
70.Why does water stop boiling when sugar is added to it when it is boiling?
71.Write the structures of the following :H2SO3
................................ Advertisement ................................
72.Write the structures of the following :XeF4
73.The α-hydrogen atoms of aldehydes and ketones are acidic in nature. Give reasons.
74.Benzoic acid does not give Friedal-Crafts reaction. Give reason.
75.Iron on reaction with HCl forms FeCl2 and not FeCl3.Why?
76.BiH3 is the strongest reducing agent amongst all the hydrides of group 15.Why?
77.Draw the structure of the following: H4P2O7 (Pyrophosphoric acid)
78.Draw the structure of the following:XeF2
79.Reducing character decreases from SO2 to TeO2.Why?
80.H2S has lower boiling point than H2O.Why?
81.Bond angle in NH4+ is higher than NH3.Why?
83.Define Activation energy.
84.Transition metals and their compounds show catalytic properties.Give reason.
................................ Advertisement ................................
85.Highest fluoride of Mn is MnF4 whereas the highest oxide is Mn2O7.
86.Write the equations involved in Stephen reaction.
87.Write the equations involved in Wolff-Kishner reduction.
88.Write the equations involved in Etard reaction.
89.How do you convert the following : Benzoic acid to Benzaldehyde.
90.How do you convert the following : Ethyne to Ethanal
91.How do you convert the following : Acetic acid to Methane.
92.Why are alkyl halides insoluble in water ?
93.Why is Butan-1-ol optically inactive but Butan-2-ol is optically active ?
94.Although chlorine is an electron withdrawing group, yet it is ortho-, para- directing in electrophilic aromatic substitution
reactions. Why ?
95.Draw the geometrical isomers of complex [Pt(en)2Cl2]2+.
96.On the basis of crystal field theory, write the electronic configuration for d4 ion, if △o > P.
97.Write the hybridization type and magnetic behaviour of the complex [Ni(CN)4]2-. (Atomic number of Ni = 28)
98.A delta is formed at the meeting point of sea water and river water.Give reason.
................................ Advertisement ................................
99.NH3 gas adsorbs more readily than N2 gas on the surface of charcoal.Give reason.
100.Powdered substances are more effective adsorbents.Give reason.
101.Indicate the principle behind the method used for the refining of Nickel.
102.What is the role of dilute NaCN in the extraction of gold ?
103.What is "copper matte" ?
104.Write the name of the vitamin whose deficiency causes bleeding of gums.
105.What is the difference between acidic amino acids and basic amino acids ?
106.Which one of the following is a monosaccharide : starch, maltose, fructose, cellulose.
107.Write the name and structure of the monomer of the following polymer :Terylene
108.Write the name and structure of the monomer of the following polymer :Bakelite
109.Write the name and structure of the monomer of the following polymer :Buna-S
110.State Kohlrausch law of independent migration of ions. Write its one application.
111.Name the reagents used in the following reaction :
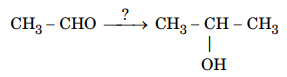
112.Name the reagents used in the following reaction :
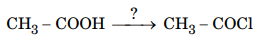
................................ Advertisement ................................
113.Write down the IUPAC name of the following complex : [Cr (en)3]Cl3
114.Write the formula for the following complex : Potassium tri oxalato chromate(III)
115.Physisorption is reversible while chemisorption is irreversible. Why ?
116.Write the IUPAC name of the given compound :
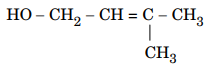
117.Which allotrope of sulphur is thermally stable at room temperature ?