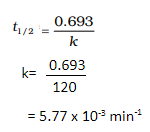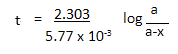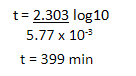Chemical Kinetics : Important Questions And Answers
List of Questions and Answers
1.The branch of chemistry, which deals with the study of reaction rates and their mechanisms, is called ______________.
Ans:Chemical Kinetics
2.What are the factors that affect the rate of reaction?
Ans: The factors affecting rate of reaction are as follows:
- Concentration
- Temperature
- Pressure
- Presence of Catalyst
- Surface Area
- Radiation
3.Define Rate of Reaction.
Ans: The rate of a reaction can be defined as the change in concentration of a reactant or product in unit time.
4.Define Rate Law.
Ans: The representation of rate of reaction in terms of concentration of the reactants is known as rate law. It is also called a rate equation or rate expression.
5.The rate constant for first order reaction is 60/s. How much time will it take to reduce the concentration of the reaction to 1/10 of its initial value.
Ans:
The first order reaction is :
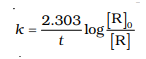
So the formula will be :
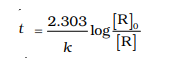
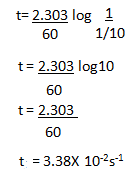
6.The initial concentration of N2O5 in the following first order reaction N2O5(g) → 2NO2(g) + 1/2O2(g) was 1.24 × 10–2 mol L–1 at 318 K. The concentration of N2O5 after 60 minutes was 0.20 × 10–2 mol L–1. Calculate the rate constant of the reaction at 318 K.
Ans:
The formula is :
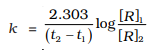
After substituting the values we get :

7.
(a) What is the order of reaction whose rate constant has the same unit as the rate of
reaction?
(b) Thermal decomposition of a compound is of first order. 50% decomposes in 120 minutes. How long will it take for 90% to decompose?
Ans:
a) Zero order reaction.
b) The half life reaction to decompose takes 120mins
The formula is